Study Finds Tubeless Insulin Delivery System Safe and Effective for People with Diabetes
As diabetes device developer Insulet works to get its Omnipod 5 FDA-approved and prepares to release it to the public by the end of 2021, a team of researchers has been working to find out exactly how safe and effective the new closed-loop system really is.
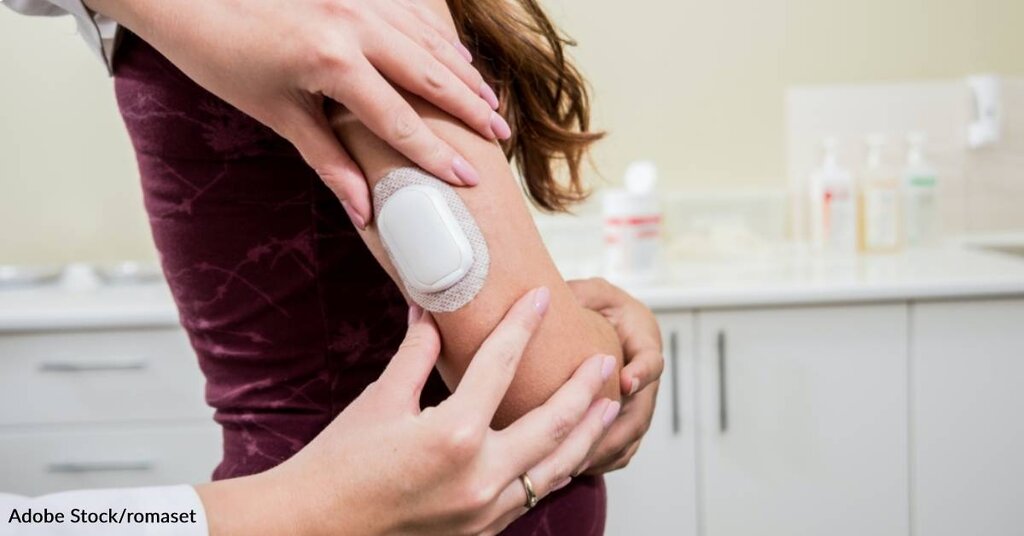
The Omnipod 5 is the first of its kind, a device that delivers insulin through a wearable and wireless pod worn on the skin. The device communicates wirelessly with a handheld Personal Diabetes Manager (PDM) called Dash, which looks and functions like a modern touchscreen smartphone but is designed to help track and deliver insulin. Dash can also connect with the user’s own smartphone via Bluetooth to give users more direct information about their blood sugar levels.
A team of researchers has studied the device using data from 240 participants with type 1 diabetes. For two weeks, the participants received standard diabetes therapies and were assessed for their blood glucose patterns. Then those results were compared with three months on the tubeless insulin delivery system.

112 of the participants were children with a mean age of 10.3. 128 of them were adults with a mean age of 36.9. A little over half of the participants were female, and just over 90 percent of them were white.
Participants used the automated mode on their devices more than 96 percent of the time. Device deficiencies were found to occur roughly once per month per person.
At the end of the study, participants saw their glycated hemoglobin reduced by 0.71 percent for adults and 0.38 percent for children on the automated system. Time spent in range increased by 9.3 percent for adults and 15.6 percent with children.

The response was faster for adults, who saw results in just three days and spent roughly 73.5 percent of their time in the recommended hypoglycemic range (<70 mg/dL) after that point. The children only spent 62.6 percent of their time in range after three days, but by four to six days, that time increased to 68 percent and remained stable afterward. Time spent in a hyperglycemic state (>180 mg/dL) was decreased by 15.1 percent for children and 7.7 percent for adults. Rates of hypoglycemia for both groups (4.8 per 100 person-years) and diabetic ketoacidosis (1.2 per 100 person-years) were lower than the average reported by the U.S. Type 1 Diabetes Exchange Registry. Children required more insulin per day on the system to stay in range, while adults actually required less.
The researchers say their data suggest that automated tubeless insulin delivery is safe and effective at stabilizing glycated hemoglobin levels among children and adults with type 1 diabetes. In fact, it can even improve glycemic control among people with diabetes.
Because of the single-arm design of the study and its short duration, more research is needed to corroborate these results. However, this is a promising start for the Omnipod 5. The study was published in the journal Diabetes Care.